Introduction: Tisagenlecleucel (tisa-cel) is a chimeric antigen receptor (CAR) T-cell therapy approved by the United States (US) Food and Drug Administration (FDA) for the treatment of patients up to 25 years of age with B-cell precursor acute lymphoblastic leukemia (ALL) that is refractory or in second or later relapse, and adult patients with relapsed or refractory large B-cell lymphoma (LBCL) after at least 2 lines of systemic therapy. Real-world data on the safety of tisa-cel are limited, particularly in the pediatric and young adult patient ALL indication. Data from the Center for International Blood and Marrow Transplant Research (CIBMTR) and Cellular Therapy (CT) Registry presented at the American Society of Hematology Annual Meeting in 2019 found that efficacy and safety outcomes in 105 patients treated with tisa-cel for the ALL indication (Grupp 2019) and 47 treated for the LBCL indication (Jaglowski 2019) were largely similar to the clinical trials. Because tisa-cel is the only CAR T-cell therapy currently approved for 2 distinct indications in 2 distinct populations, we sought to further characterize the real-world adverse event (AE) profile of tisa-cel in each indication. In this large-scale analysis, we utilized the FDA Adverse Events Reporting System (FAERS) database to assess tisa-cel-related AEs in both the ALL and LBCL indications.
Methods: The FAERS database contains de-identified reports of product-related AEs, coded using the Medical Dictionary for Regulatory Activities (MedDRA) and classified as serious or non-serious. The database was queried for cases involving tisa-cel (and its trade name) in patients up to 25 years of age for the ALL indication, from the FDA approval date (August 30, 2017) through March 31, 2020; and in patients age ≥18 years for the LBCL indication, from the FDA approval date (May 1, 2018) through March 31, 2020. Cases were excluded if the age of the patient was unknown, or if the case was reported outside the US. Patient characteristics and AEs were summarized using descriptive statistics; comparisons of the proportion of AEs by indication were made using the Chi-square test with statistical significance determined at a two-sided α=0.05.
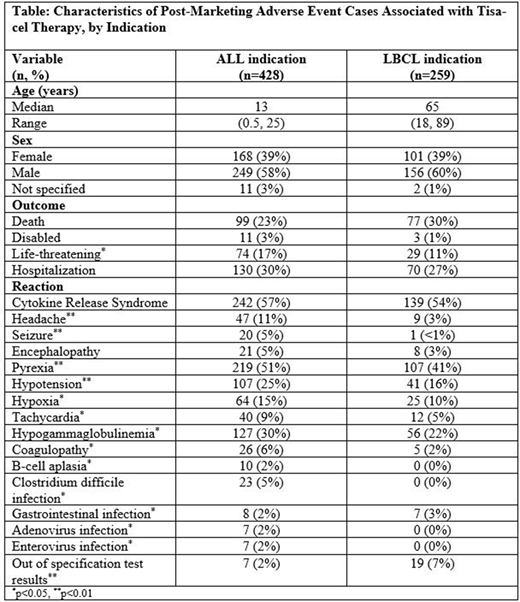
Results: A total of 687 cases were retrieved (428 pediatric or young adult ALL, 259 adult LBCL); 94% of the cases were classified as serious. The median age among ALL cases was 13 years (range 0.5-25 years) and the median age among LBCL cases was 65 years (range 18-89 years) (Table). For both indications, cytokine release syndrome (CRS) was the most commonly reported reaction term (56.5% of ALL cases and 53.7% of LBCL cases). Some clinical features of CRS, including pyrexia, hypotension, and tachycardia, were reported more frequently among ALL cases compared with LBCL cases (pyrexia 51% vs. 41%; hypotension 25% vs. 16%; and tachycardia 9% vs. 5%, for ALL versus LBLC cases, respectively). A greater proportion of ALL cases reported immune effector cell-associated neurotoxicity syndrome (ICANS), including headache and seizure (11% and 3% respectively, vs. 3% and <1% in LBCL cases). Hypogammaglobulinemia and infections were also reported in ALL cases more often than in LBCL cases. Out of specification test results with tisa-cel were reported in a greater proportion of LBCL cases (1.6% of ALL cases vs. 7.3% of LBCL cases).
Conclusion: In this, the largest real-world analysis of AEs associated with tisa-cel to date, we found statistically significant differences in AE patterns between ALL and LBCL. A greater proportion of cases under the ALL indication reported ICANS, infections, and some CRS clinical features, including pyrexia, hypotension, and tachycardia. Out of specification test results showed trends similar to those reported from the CIBMTR CT Registry, where a higher proportion of LBCL patients had cell viability <80% compared to ALL patients. These differences in reported AEs based on the indication can complement clinical trial data and can assist clinicians in appropriate prevention and mitigation efforts when using tisa-cell.
Zettler:Cardinal Health: Current Employment. Feinberg:Cardinal Health: Current Employment. Balanean:Cardinal Health: Current Employment. Gajra:Cardinal Health: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal